Classify Each Property as Physical or Chemical
The temperature at which dry ice evaporates c. Classify each property as physical or chemical.

Physical And Chemical Changes Write And Draw Worksheet Teaching Chemistry Chemistry Worksheets Physical Science
The color of gold.

. View physical and chemical properties and changes WSdocx from CHEMISTRY 1 CHEMISTR at Maurice J. The shine of silver c. Physical properties such as hardness and boiling point and physical changes such as melting or freezing do not involve a change in the composition of matter.
And to change what it is. Sodium is a soft metal can be cut with a knife Physical Property b. Intensive properties are independent of the amount of matter present.
From the above we can therefore say that the answer The classification of the following elements as a physical or chemical property is shown below. Physical property because the metal is just getting shinier not creating a new substance B. Thats a chemical property.
Doil and water do not mix. All substances have distinct physical and chemical properties and may undergo physical or chemical changes. View the full answer.
Recall that physical properties can be observed without producing new substances. Physical property because the substance is changing form not creating a new substance C. Chemical properties such flammability and acidity and chemical changes such as rusting.
Chemical properties describe how a substance interacts with other substances to produce new substances. The boiling point of ethyl alcohol b. The odor of paint thinnerd.
100 19 ratings Ans. The tendency of iron to rust d. Whereas a property which brings change in chemical composition of a substance is known as a chemical property.
Reacts with a base to form water physical 5. The flammability of propane gas. A property which brings no change in chemical composition of a substance is known as a physical property.
All substances have distinct physical and chemical properties and may undergo physical or chemical changes. The tendency of ethyl alcohol to burnb. For example size mass density etc are physical property.
Can neutralize a base physical property 3. Classify the six underlined properties in the following paragraph as chemical or physical. - The color of copper wire - The reactivity of iron with oxygen - The luster of silver jewelry - The explosiveness of fireworks - The flammability of ether - The melting point of water - The smell of ginger.
Physical or chemical properties 1. Bitter taste physical taste 2. Biron is more dense than aluminum.
The shine on silverc. The tendency of silver to tarnish. In this there are two other classifications are intensive and extensive properties.
Classify each property below as extensive physical intensive physical or. Physical properties are aspects of a cetain compound that can be observed or measured without changing the composition of the molecule. Chemical Property c.
Extensive properties depend upon the amount of matter in the sample. Sodium reacts violently with water to produce hydrogen gas and sodium hydroxide. All substances have distinct physical and chemical properties and may undergo physical or chemical changes.
Classify the six underlined properties in the following paragraph as chemical or physical. Fluorine is a pale yellow gas that reacts with most substancesThe free element melts at 220 C and boils at 188 CFinely divided metals burn in fluorine with a bright flameNineteen grams of fluorine will react with 10 gram of hydrogen. Therefore physical changes include cutting a substance into smaller pieces melting freezing evaporating etc.
The shine of chrome. Label each of the following properties of sodium as either a physical property or a chemical property. Chemistry End of Chapter Exercises.
Find step-by-step Chemistry solutions and your answer to the following textbook question. Physical property on the other hand is that you can change it but it doesnt change what it is. Chemistry End of Chapter Exercises.
Classify each property as physical or chemicala. Okay Chemical properties means that you change the composition of something or something has ability to change its composition. Chemical properties such flammability and acidity and chemical changes such as rusting.
The flammability of propane gas. Physical properties such as hardness and boiling point and physical changes such as melting or freezing do not involve a change in the composition of matter. Classify each property as physical or chemical.
Physical properties such as hardness and boiling point and physical changes such as melting or freezing do not involve a change in the composition of matter. Classify each property as physical or chemical. Boils at 88 degrees C.
Intensive properties are char View the full answer. Classify each property as physical or chemical. C magnesium burns brightly when ignited.
You can change its state change its form. Boiling point physical property 4. The odor of paint thinner d.
Chemical changes occur when a substance is converted to a different substance. When exposed to air sodium forms a white oxide. The physical properties of substances include their state of matter solid liquid or gas size shape appearance etc.
The tendency of ethyl alcohol to burn b. Classify each of the properties as a physical property or a chemical property. Fluorine is a pale yellow gas that reacts with most substancesThe free element melts at 220 C and boils at 188 CFinely divided metals burn in fluorine with a bright flameNineteen grams of fluorine will react with 10 gram of hydrogen.
The flammability of propane gas. Physical properties Chemical properties Answer Bank conductivity melting point flammability boiling point color susceptibility to rust. The color of gold.
Emercury melts as -39 C. We review their content and use your feedback to keep the quality high. Chemical properties such flammability and acidity and chemical changes such as.
A iron and oxygen form rust.

Properties Of Matter Poster Properties Of Matter Science Poster Physical Properties

Properties Of Matter Foldable Notes And Sort Digital And Print Activity Properties Of Matter Physical Properties Of Matter Elementary Science Classroom
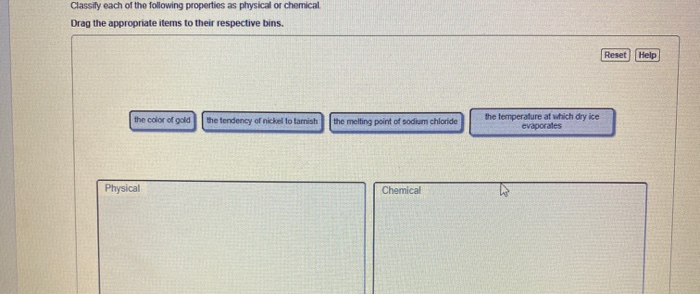
Solved Classify Each Of The Following Properties As Physical Chegg Com
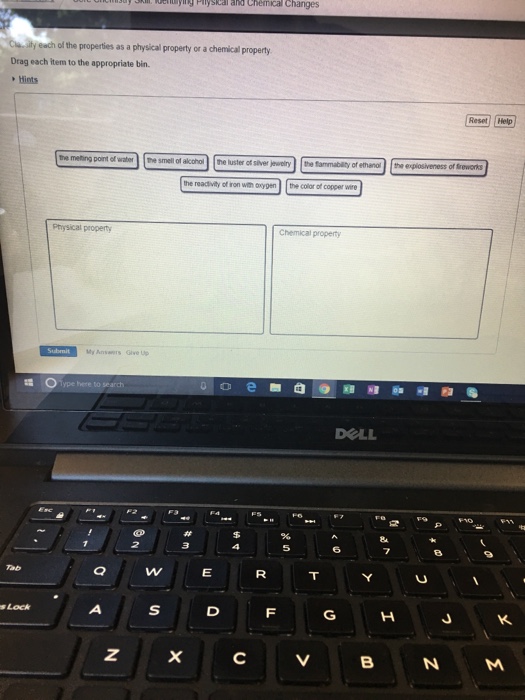
Solved Classify Each Of The Properties As A Physical Or A Chegg Com
No comments for "Classify Each Property as Physical or Chemical"
Post a Comment